SARS-CoV-2 Antibody Assays
SARS-CoV-2 Neutralizing Antibody ELISA Kit:
Evaluation of immune response after COVID-19 vaccination and infection.
- Quantitative, high throughput analysis for neutralizing antibodies in blood samples.
- Particularly suitable for determining the neutralizing antibody titer after COVID-19 vaccination.
- Neutralizing antibodies resulting from vaccination blocks the invasion of virus into host cells.
- The ELISA specifically quantifies the neutralizing antibody titer of those antibodies, which will:
- Block the receptor-binding domain (RBD) of the S1 subunit of virus’ Spike protein.
- Thereby preventing the S1-RBD attachment to the human host cell ACE2 receptor.
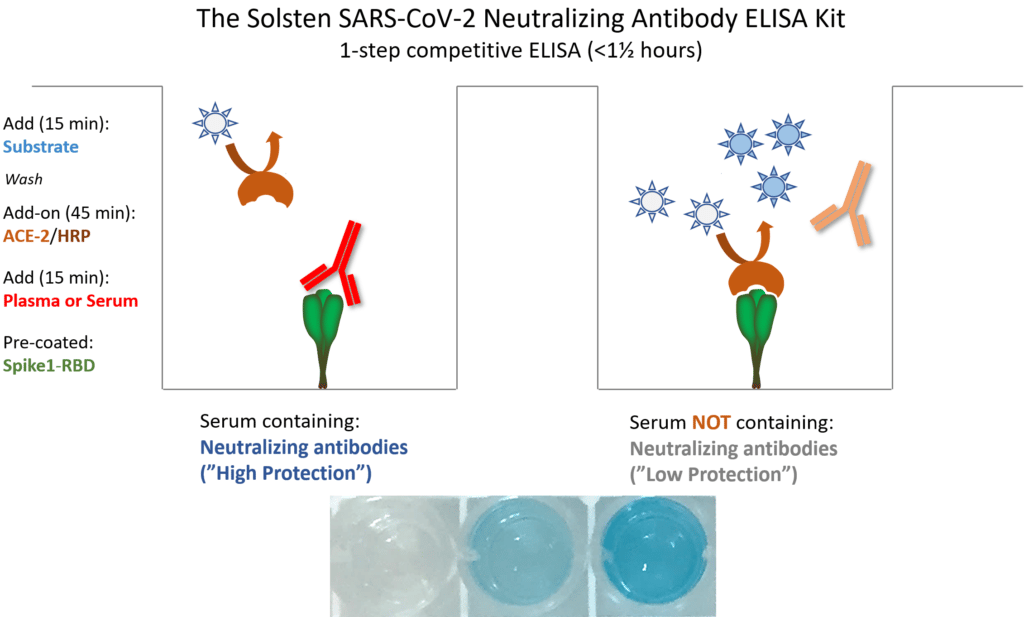
This is achieved in a 1-step competitive ELISA based on:
- ELISA-wells precoated with S1-RBD
- Simultaneous competitive incubation of blood sample (antibodies) and peroxidase-labeled ACE-2
It takes little manual labor to analyze hundreds of samples in one run.
- Clinically documented, CE-IVD marked ELISA kit including all necessary reagents.
1-step competitive ELISA
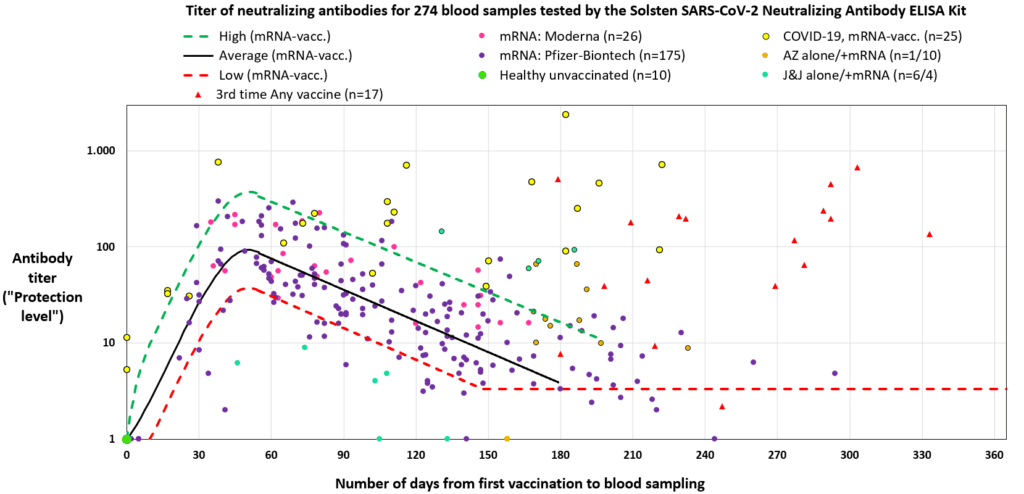
The Solsten SARS-CoV-2 Neutralizing Antibody ELISA Kit is a useful tool for quantifying the individual, time-dependent response to vaccination with COVID-19 vaccines from Pfizer-Biontech, Moderna, Astra Zeneca and Johnson&Johnson. Reference: Finalized study per January 11, 2022 at Solsten Diagnostics.
The Solsten SARS-CoV-2 Neutralizing Antibody ELISA is a 1-step competitive assay, where neutralizing antibodies in the blood sample competes with the peroxidase-labeled human ACE-2 receptor to bind to the immobilized receptor-binding domain of the virus’ Spike protein (S1-RBD). The reduction in color signal is proportional to the antibody-derived protection (titer) against the virus.
SARS-CoV-2 S&N-IgM/IgG Antibody Rapid Test (Colorimetric)
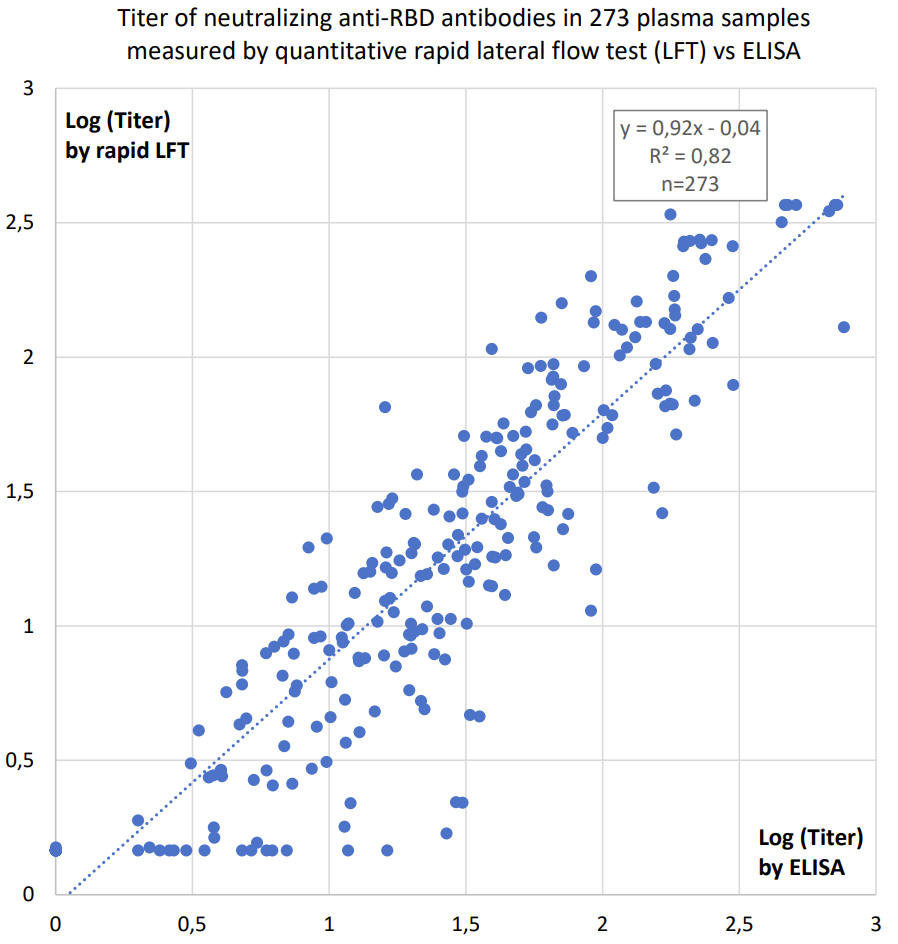
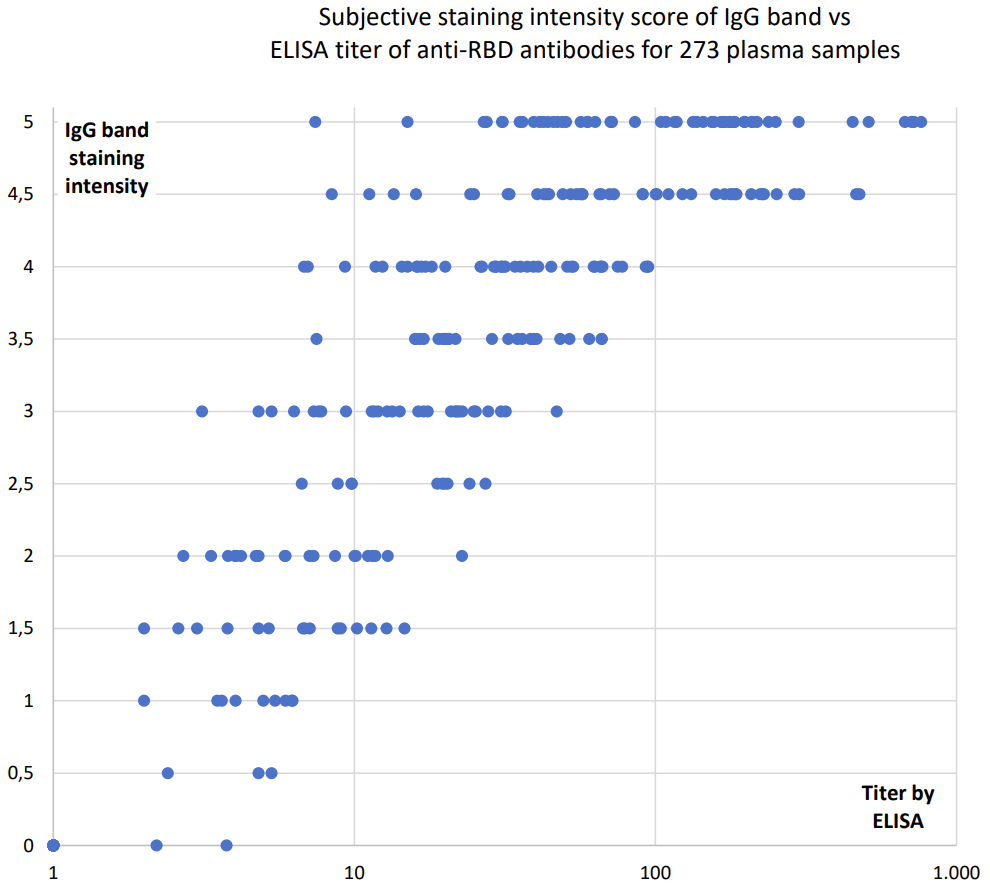
The SARS-CoV-2 S&N-IgM/IgG Antibody Rapid Test is done is in 10 minutes, and the optional two bands for IgG and IgM antibodies against the SARS-CoV-2 spike or nucleocapsid antigens are read easily by eye. Though the test is qualitative, the intensity of the IgG-band indicates the titer of the blood sample (according to comparisons with ELISA quantification, see graph below).
- Easy-to-use Rapid Test for IgG and IgM antibodies reacting with antigens (N and S) of SARS-CoV-2.
- Apply one drop of blood (fingerprick full blood or serum/plasma from venous blood sample).
- Results in 10 minutes and qualitative reading by eye (pregnancy-test like output).
- Clinically documented, CE-IVD marked Quick test kit for immediate use.
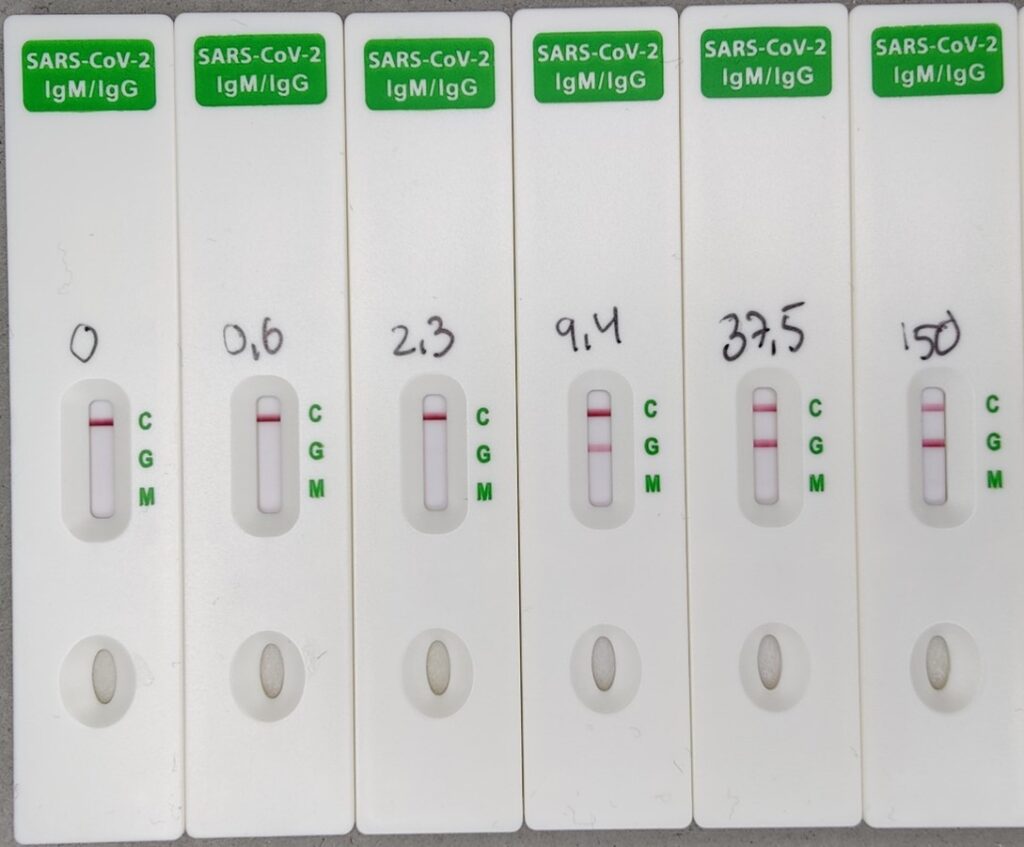
The SARS-CoV-2 S Antibody Quantitative Rapid Test
The SARS-CoV-2 S Antibody Quantitative Rapid Test is done in 15 minutes, and the titer of antibodies against the Spike (S) protein of SARS-CoV-2 is measured as a fluorescence signal by the Fluorescence Immunoassay Analyzer
- Easy-to-use Quantitative Rapid Test for antibodies reacting with the receptor-binding domain (RBD) of the spike (S) protein of SARS-CoV-2.
- Use one drop of blood (20 µl of fingerprick full blood or 10 µl of serum/plasma from a venous blood sample).
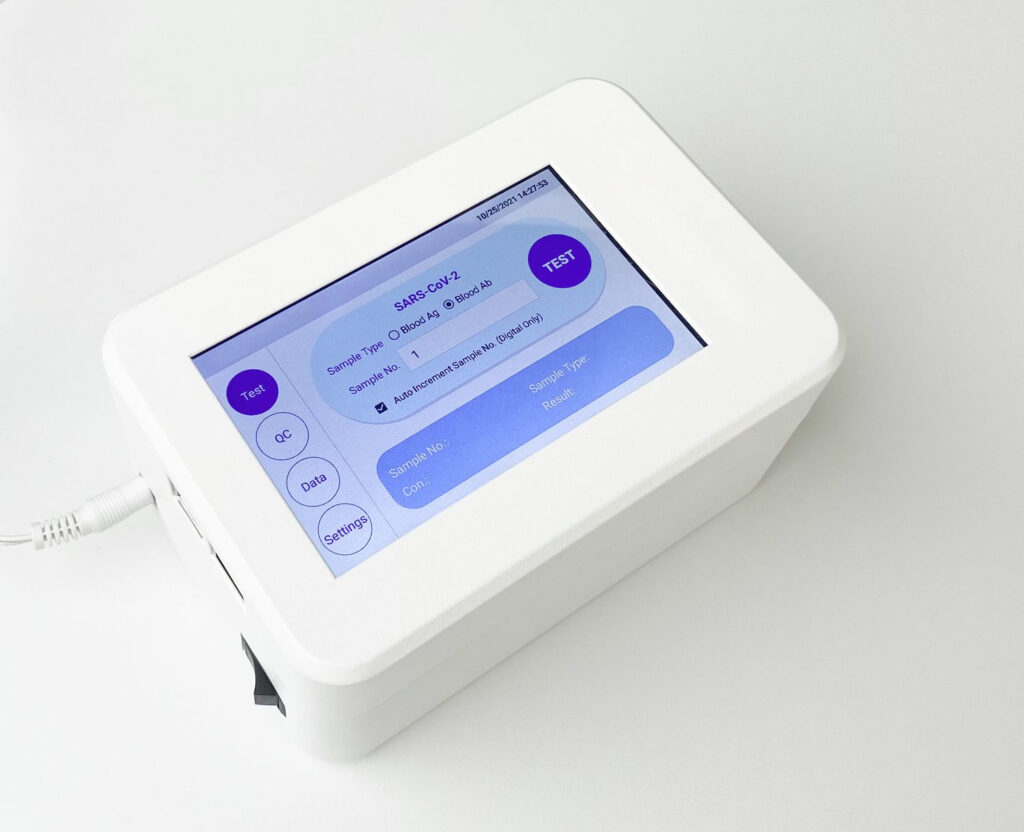
- The result is achieved in 15 minutes including quantitative reading in 20 seconds by the Fluorescence Immunoassay Analyzer Instrument.
- Clinically documented, CE-IVD marked quantitative rapid test (see correlation to ELISA results below).
- Reading of test result requires the Fluorescence Immunoassay Analyzer Instrument
- Read about the instrument