COVID-19 Antigen Assay
SARS-CoV-2 Antigen ELISA Kit:
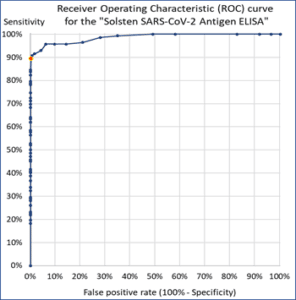
The Solsten SARS-CoV-2 Antigen ELISA Kit is a CE-marked in vitro diagnostic product with unique characteristics:
- It quantifies the nucleocapsid protein antigen of SARS-CoV-2 in blood samples
- It demonstrates COVID-19 at the same early stage of infection as PCR-analysis
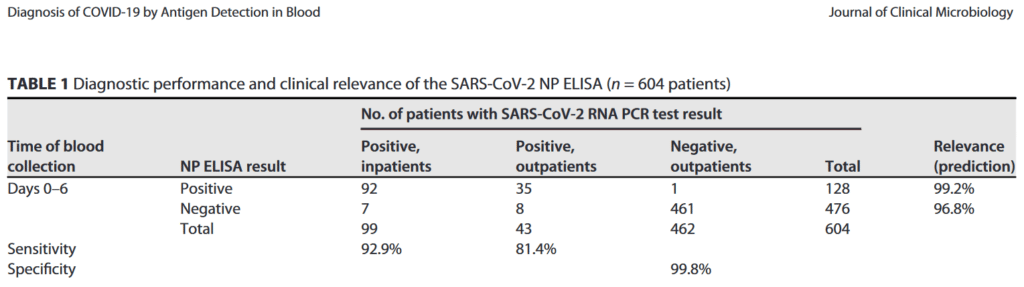
- It has an accuracy, specificity and sensitivity at the level of PCR-analysis of upper respiratory swabs
See recent peer-reviewed publication in J. Clinical Microbiology:
These characteristics makes the Solsten SARS-CoV-2 Antigen ELISA Kit particularly suitable for:
Screening for SARS-CoV-2 hospital (nosocomial) infections, since:
- The blood samples (serum or plasma) have already been collected for other analytical purposes
- Additional sampling by staff is avoided.
- One technician can analyse several hundred blood samples by ELISA in one batch-run.
- The analysis time of the ELISA is 2 hours per batch-run, and it is easy to automate.
- The price of ELISA-screening is at least 4x lower per patient than testing by PCR.
The SARS-CoV-2 N Antigen Quantitative Rapid Test

The SARS-CoV-2 N Antigen Rapid Test is done in 15 minutes, and the concentration of SARS-CoV-2 nucleocapsid (N) antigen is measured as a fluorescence signal by the Fluorescence Immunoassay Analyzer.
- Easy-to-use Quantitative Rapid Test for the N antigen in blood.
- Use one drop of blood (20 µl of fingerprick full blood or 10 µl of serum/plasma from a venous blood sample).
- The result is achieved in 15 minutes including quantitative reading in 20 seconds by the Fluorescence Immunoassay Analyzer Instrument
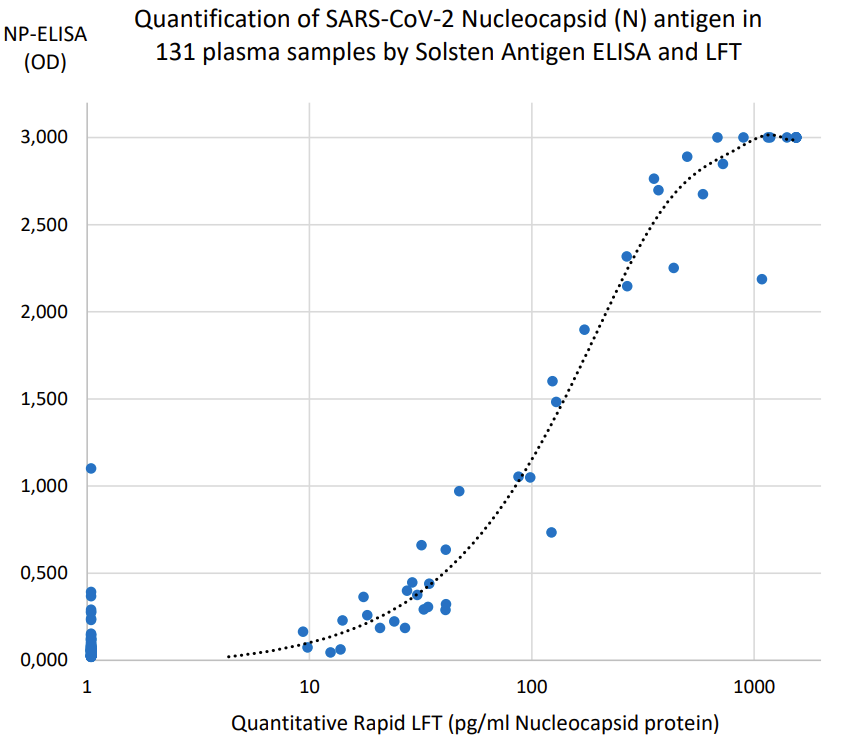
- Clinically documented, CE-IVD marked quantitative rapid test (see correlation to ELISA results below).
- Reading of test result requires the Fluorescence Immunoassay Analyzer Instrument.
Read more about Fluorescence Immunoassay Analyzer Instrument